By Linda Conlin, Pro to Pro Managing Editor
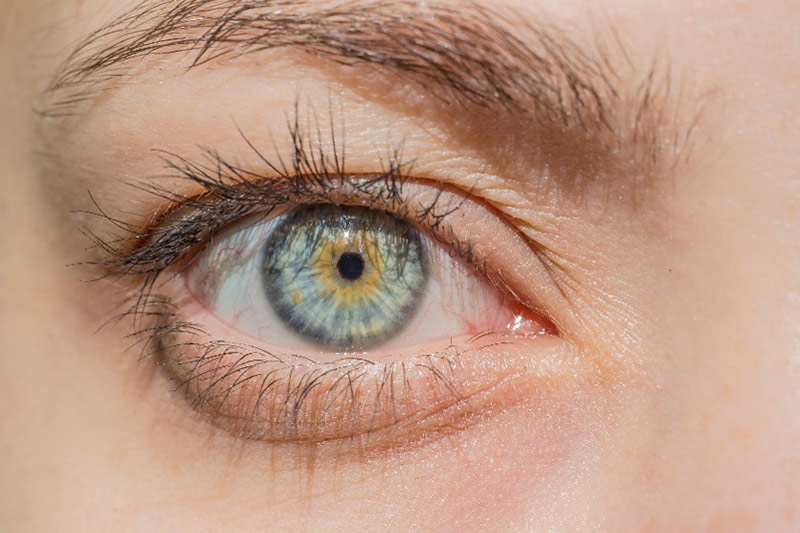
The FDA sent a warning letter to LightEyez Limited, located in the UK, regarding their OTC eye lightening drops and other of the company’s ophthalmic drug products. The FDA tested ten samples of MSM Eye Repair Drops, which the company claims, “Speeds up Eye Color Change,” manufactured at the company’s Sarasota, Florida facility. The FDA tests found microbial contaminants including Pseudomonas spp., Mycobacterium spp., Mycolicibacterium spp., and Methylorubrum spp. The FDA noted in the warning, “Using contaminated eye drops could result in a range of ocular infections, from minor to serious vision-threatening infections which could progress in some cases to a life-threatening infection.”
The Sarasota manufacturer confirmed that the drug components were imported from LightEyez and added that “a malfunctioning water system resulted in the use of unfiltered municipal water in (the) ophthalmic drug product.” The FDA warning letter also expressed concerns that the company website notes that the drug containers may leak during shipping, and in addition instructs consumers to clear blockages in the droppers by inserting “a foreign object into the dropper tip nozzle and/or remove the dropper tip to clear a blockage, which risks contamination of (the) ophthalmic drug products with foreign particulate matter and/or microorganisms.” (https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/lighteyez-limited-665450-02152024)
The drops lighten eye color by inhibiting the production of melanin, which gives pigmentation to the iris. The pigmentation of the iris varies from light to dark, depending on the concentration of melanin in the iris pigment epithelium, located on the back of the iris. Just as melanin protects the skin from photodamage, it also protects the eye. Those with grey, blue, and green eye colors, as well as albinos, have more sun-related ocular issues. Melanin also is found in the retinal pigment epithelium (RPE), a single layer of epithelial cells located posterior to the photoreceptors in the retina. While it is not clear whether the eye lightening drops also inhibit melanin in the RPE, the reduction of melanin in the iris allows more UV to reach the retina.
The FDA began its investigation in April 2023 with the batch testing. In August 2023, the FDA issued a drug safety alert warning consumers not to purchase or use certain of these eye drops due to bacterial contamination. Following a teleconference with LightEyez’ legal representative in the U.K., also in August 2023, Light Eyez ceased communication with the FDA. Currently, no eye color changing eye drops are FDA approved. On August 28, 2024, the American Academy of Ophthalmology issued a warning about the drops due to the attention the drops have been receiving on social media. According to JoAnn A. Giaconi, MD, clinical spokesperson for the American Academy of Ophthalmology, “The ads show dramatic ‘before-and-after’ shots (with) vague information on how the drops actually work to change eye color,” she said in the news release. “But here’s the reality, there’s no evidence that they do anything at all, and no evidence that they’re safe.”